-
Aging
Science Saturday: Neutrophils may lead to cell aging
The action of an immune cell called a neutrophil may contribute to tissue aging, according to a new study. As neutrophils do their job, they may damage chromosomes in nearby cells which, over time, may lead to cellular senescence. This new concept may help clarify cell aging. It also suggests targeting neutrophils might be helpful in treatment of age-related disease.
Neutrophils, Senescent Cells
The immune system is a collection of cells and proteins that works to keep the body healthy. But it's a balancing act. Tip in one direction and an infection might cause organ damage or lead to sepsis. Overbalance in the other and the cure might lead to an autoimmune disease. Neutrophils are a key part of immune system action. They help healing by clearing out cellular debris after an infection. They're also armed and can kill microbes. During infection, neutrophils release a short blast of unstable molecules called reactive oxygen species.
Another way the immune system keeps the body healthy is by telling damaged cells to perish. But not all cells die. Cells told to close down by the body sometime ignore that signal. Instead, they live in a sort of zombie state, undead but spewing toxic chemicals. These cells are senescent cells. They damage their neighbors through release of a toxic protein stew.

The body has many reasons to tell a cell to expire: it's time for a hand to separate into fingers in an embryo, the cell has reached its limit on division, or the cell is on a pathway that leads to cancer. Senescent cells are the ones that refuse the body's order to expire. These cells are implicated in conditions of aging, ranging from osteoporosis to diabetes and muscle weakness. Mayo Clinic researchers are in the forefront of these investigations into how senescent cells contribute to illnesses and what might be done to treat them.
Neutrophils and Collateral Damage to Telomeres
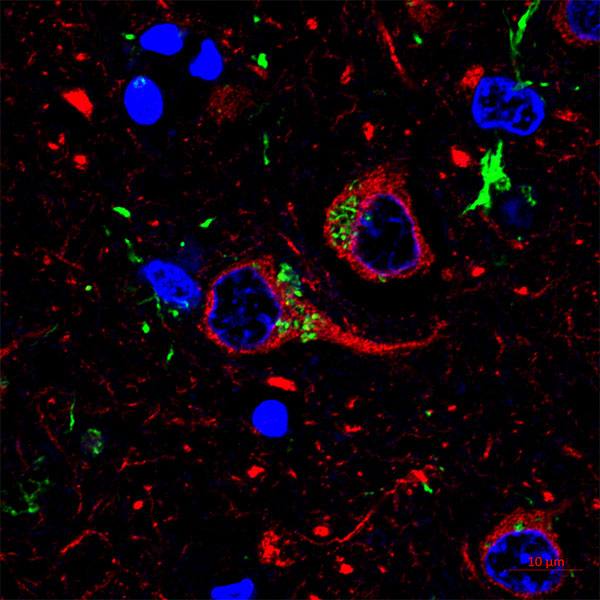
Mayo Clinic scientist João Passos, Ph.D., and an international team of colleagues examined neutrophils' effect on human and mouse cell senescence. They co-cultured neutrophils with human cells and depleted neutrophils in mice to determine their role in encouraging senescence.
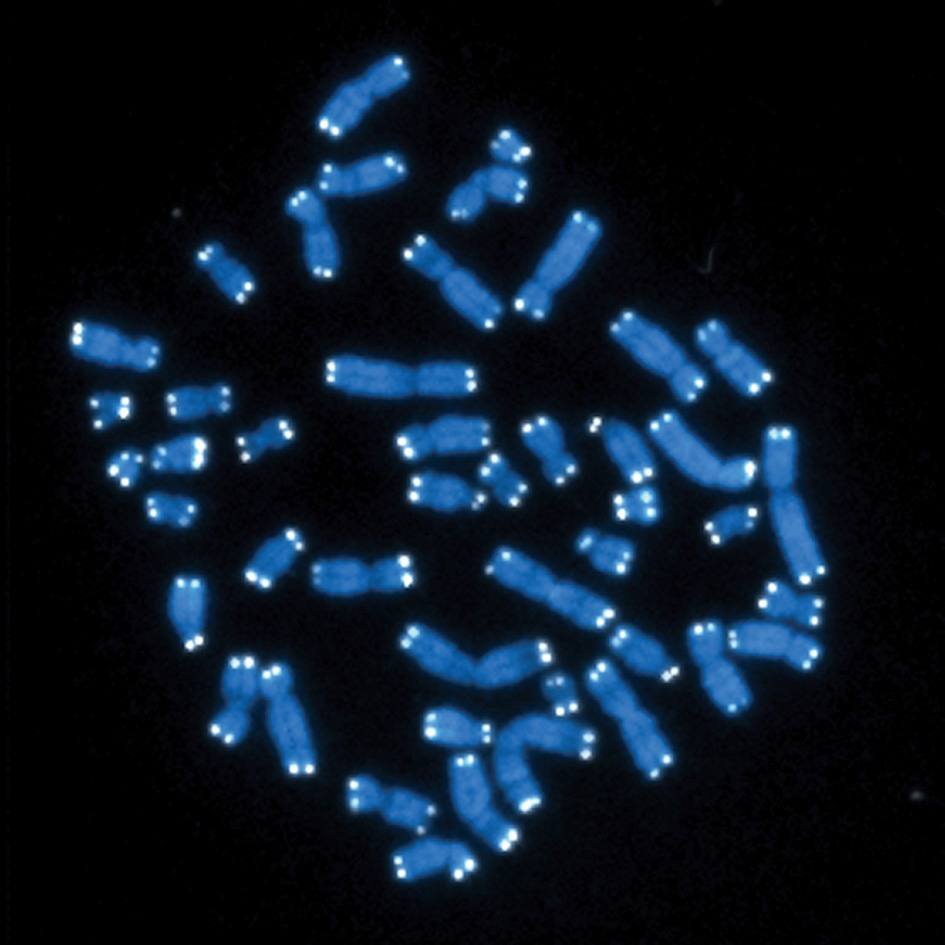
"We asked if neutrophils could be drivers of cellular senescence in tissues and contribute to aging," Dr. Passos explains. "We found that neutrophils can cause senescence in neighboring non-immune cells by damaging their telomeres via reactive oxygen species. We also found that if we deplete neutrophils in mice, we can prevent telomere damage and senescence."
Telomeres are DNA structures found at the ends of chromosomes. They protect chromosomes from damage during copying. In previous research on telomeres in skin cells, Dr. Passos likened them to the canary in the coal mine. Telomeres are sensitive to stress, so damage signals to a cell that something is wrong. That signal may direct the cell to enter senescence. The current finding, in The EMBO Journal, extends previous work Dr. Passos says, by clarifying how telomeres are damaged.
"Our study suggests an evolutionary trade-off between having a good working immune system and age-related pathology. While neutrophils have evolved to play important roles in fighting infection, they may contribute to collateral damage and induction of cellular senescence which will be detrimental later in life," he says.
The paper shows that neutrophils can induce damage to telomere regions of the genome. Neutrophils, as the authors write, may be both recruited by senescent cells and can be drivers of senescence. Their results are consistent with the idea that appearance of a few senescent cells may transmit senescence to otherwise healthy cells via the recruitment of neutrophils.

Next Up: Limit the Damage?
The authors plan to continue investigating the role of neutrophils in aging. Dr. Passos and team are particularly interested in potential therapies for age-related disease prevention.
"We want to see if clearance of neutrophils is beneficial to healthspan in mice and prevents senescence during normal aging," says Dr. Passos.
Funding for this research comes, from benefactors and the National Institutes of Health, including a grant from the National Center for Advancing Translation Sciences. Mayo Clinic owns a patent on INK-ATTAC mice. For the complete list of authors, funders and conflicts, see The EMBO Journal.
- To read more about senescent cell research on Discovery's Edge, head over to the search page: Senescent Cells
- For more about skin aging, read, "Researchers Identify Cells that Drive Human Skin Aging."
- For more about SASP, read, "Toxic Ooze Linked to Biological Age, Disease and Disability."
- For more about immune system aging, read, "Aging and Immune Decline."