-
Aging
Researchers Identify Cells that Drive Human Skin Aging
Mayo researchers report for the first time that melanocytes in skin drive aging of that organ. The information clarifies a mechanism that drives aging in human skin and may offer new ways to protect it.
Over time, cells in the body can be damaged by external exposures, like ultraviolet radiation from the sun, or internal ones like oxidative stress. On the skin this appears as wrinkles, dryness or age spots. In the skin, changes occur so the outermost layer called the epidermis gets less nourishment, becomes thinner and is easier to breach. To understand this process on a cellular level, Mayo researcher João Passos, Ph.D., and his team began looking at different cell populations in skin to see if any cell type was associated with skin damage more so than another.

They were looking at a particular structure made of DNA and protein found at the ends of every chromosome in cells, called telomeres. Dr. Passos’ team research shows that once telomeres have been damaged, they are difficult to repair, and can kick off a process that prevents cells from dividing further, called senescence.
“Telomeres are a bit like canaries in coal mines,” says Dr. Passos. “They are extremely sensitive to stressors, they warn the cells that something is going wrong and activate the senescence program.”
The team initially thought that one type of cell that is abundant in skin and divides often, called keratinocytes, would drive senescence. However they report in The EMBO Journal that melanocytes, the cells which produce the pigment responsible for skin color, fit the senescence profile and released pro-inflammatory factors that could affect surrounding cells and induce skin aging.
“Melanocytes divide very little throughout our life and constitute 5 to 10% of the cells in the basal layer of the epidermis,” says Stella Victorelli, Ph.D., the first author of the paper. “They showed a variety of molecular markers of cellular senescence in the aging skin. We found that melanocytes became senescent without telomere shortening, which is not surprising since they hardly divide. But melanocytes showed DNA damage specifically at telomere regions irrespectively of their length due to oxidative stress.”
To confirm that melanocytes were really the driver of skin aging, the team built a 3D human epidermis in the lab, and found that melanocytes alone could induce several features of skin aging in the model. They also reported that the effect of the senescent melanocytes could be moderated by treating the model with senolytic drugs or an antioxidant that protects mitochondria.

The implications of this work will help move the science of aging forward, says Dr. Passos.
“The most popular explanation to explain senescence during aging is the idea that telomeres shorten every time a cell divides, and when they reach a limit and become too short the cell is shut down and becomes senescent,” he explains. “Our research indicates that cells that do not divide regularly — such as melanocytes — can also become senescent despite not showing any age-dependent telomere shortening.”
“It also led to the development of several methodologies which allow us to precisely evaluate the burden of senescent cells in human skin. Skin is a readily accessible tissue and we believe that detection of the amount of senescent cells can be used as a prognostic tool to evaluate disease stage as well as the efficacy of treatments in clinical practice.”
This study is the first to show that:
- Melanocyte senescence increases with age.
- Melanocytes accumulate telomere damage but not telomere shortening during aging.
- Senescent melanocytes limit the proliferation of surrounding cells, contributing to skin aging.
- Clearance of senescent melanocytes improves skin aging.
This research is important, says Dr. Passos, because mechanisms that drive the aging process differ.
“Aging is characterized by multimorbidities and is the major risk factor for all age-related diseases,” he says. “By understanding the molecular mechanisms driving the aging process, we may be able to find new ways to target the aging process and delay the onset of not only one, but all age-related diseases and significantly improve the quality of life of older people.”
Funding for the study was provided by Unilever, the Biotechnology and Biological Sciences Research Council, the Glenn Foundation for Medical Research, the Academy of Medical Sciences, Mayo Clinic Center for Clinical and Translational Science and Mayo Clinic Robert and Arlene Kogod Center on Aging.
Three authors report that they are employees of Unilever and state, “although no products were tested, this work could potentially promote the use of anti-aging products and lead to financial gain for Unilever.” Other authors on the paper include collaborators from Leiden University Medical Center, Max Planck Institute for Biology of Ageing, University of Glasgow, Sanford Burnham Prebys Medical Discovery Institute, Unilever, Vanderbilt University Medical Center, and Newcastle University.
In addition to Dr. Passos, Mayo authors include first author, Stella Victorelli, Ph.D., and:
- Anthony Lagnado, Ph.D.
- Mikolaj Ogrodnik, Ph.D.
- Diana Jurk, Ph.D.
- Alexander Meves, M.D.
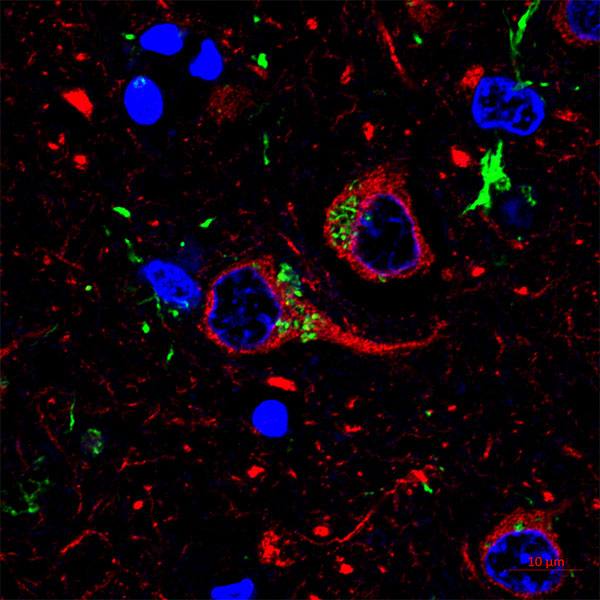
To visualize what is happening in the nucleus of a senescent melanocyte, the @PassosLab used a technique called Immuno-fluorescence in situ hybridization that causes different parts of the cell to glow. This allows the team to visualize the telomeres (red), telomere damage (yellow) and DNA damage (green). The purple area is staining for a marker of melanocytes (which is located in the cytoplasm) and the dark circular area in the middle is where the nucleus is located. To see this video in full screen please watch on YouTube: https://www.youtube.com/watch?v=34tSAY9I_Vc