-
Research
Science Saturday: Early research toward a cell-free solution for stress urinary incontinence
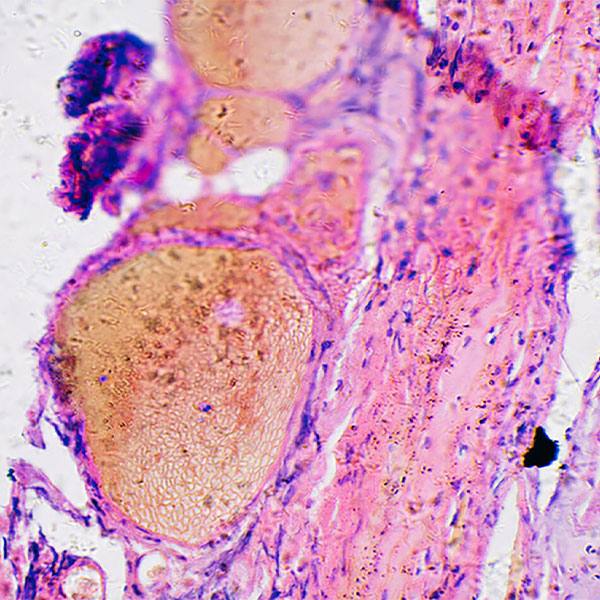
Mayo Clinic researchers found a noncellular substance suggested improvement in restoring muscle function and bladder control in preclinical models. The teams of Atta Behfar, M.D., Ph.D. and Emanuel Trabuco, M.D., led this research in a collaboration between Mayo Clinic Departments of Cardiovascular Medicine and Obstetrics and Gynecology. The paper is published in NPJ Regenerative Medicine.
"Surgical treatment for stress urinary incontinence, a condition afflicting 25 million women, has significantly declined over concerns about negative side effects," says Dr. Trabuco. “This has led many women to delay therapy and suffer needlessly. We hope to develop a minimally invasive, noncellular, exosome-based approach to muscle regeneration for urinary incontinence that not only targets the underlying cause of the condition but also avoids the problem with invasive surgical options presently available."
The research team used regenerative purified exosome product, known as PEP, derived from platelets to deliver messages into the cells of preclinical models. Exosomes are extracellular vesicles that are like a delivery service moving cargo from one cell to another, with instructions for targeting exact tissues that need repair. The study suggests that the use of purified exosome product alleviates stress urinary incontinence from musculoskeletal breakdown in animals. The team did not detect any infection or off-target toxicity with application of PEP.
"Skeletal muscle degeneration is a major cause of morbidity. Our research seeks to advance development of off-the-shelf technologies to regenerate skeletal muscle. Our hope is to discover new therapeutic options for human health and could have implications on orthopedics and reconstructive surgery," says Dr. Behfar.
Dr. Behfar is director of the Mayo Clinic Van Cleve Cardiac Regenerative Medicine Program and co-director for Innovation in Biologics for the Department of Cardiovascular Medicine.
Clinical trials will be needed to verify safety and effectiveness of using exosomal products for stress urinary continence in humans. Clinical trial testing of exosome-based therapies is an essential part of the process, as determined by the Food and Drug Administration, to prove the safety and benefit of technologies before they are available use in daily clinical care.
This study was funded in part by Mayo Clinic's Center for Regenerative Biotherapeutics and also with support from the Michael S. and Mary Sue Shannon Family and the Mayo Clinic Van Cleve Cardiac Regenerative Medicine Program. Mayo Clinic and Dr. Behfar have a financial interest in the technology referenced in this article.
—Susan Buckles